I had several questions about the new COVID-19 vaccines but found it surprisingly difficult to find the detailed information I was seeking in a consolidated place. This three-part blog synthesizes some of the information I found informative. I focus mostly on the biology of the new vaccine technologies, including mRNA, recombinant viral vector, and recombinant DNA plasmid vector vaccines.
Part I: A brief history

Since the days of Edward Jenner, vaccine science has come a long way. Jenner, an 18th-century English physician, observed that cowpox’s mild disease, called vaccinia, seemed to confer some protection against the deadlier smallpox. To test the hypothesis, he inoculated people with material from cowpox pustules and exposed them to the smallpox virus. Jenner’s work laid the foundation for modern immunology and vaccines.
Until only recently, modern vaccine strategies have also relied on the inoculation of healthy people with weakened or attenuated strains of pathogens to protect against disease. Examples of live, attenuated vaccines include the measles, mumps, and rubella combination vaccine and the chickenpox vaccine. Flu and polio shots are examples of inactivated vaccines. Other vaccines use components of pathogens, such as proteins or sugars. The hepatitis-B, HPV, and shingles vaccines fall under this category. Finally, toxoid vaccines contain disease-causing toxins, such as those found in diphtheria and tetanus. All but the live vaccines use adjuvants, compounds added to boost the immune response and enhance vaccine effectiveness.

The history of vaccines provides some context for the remarkable story behind this moment in science. The new technologies for the COVID-19 vaccines were not developed overnight but relied on the wisdom that comes from years of trial, error, and refinement. Vaccine science was primed to meet this moment.
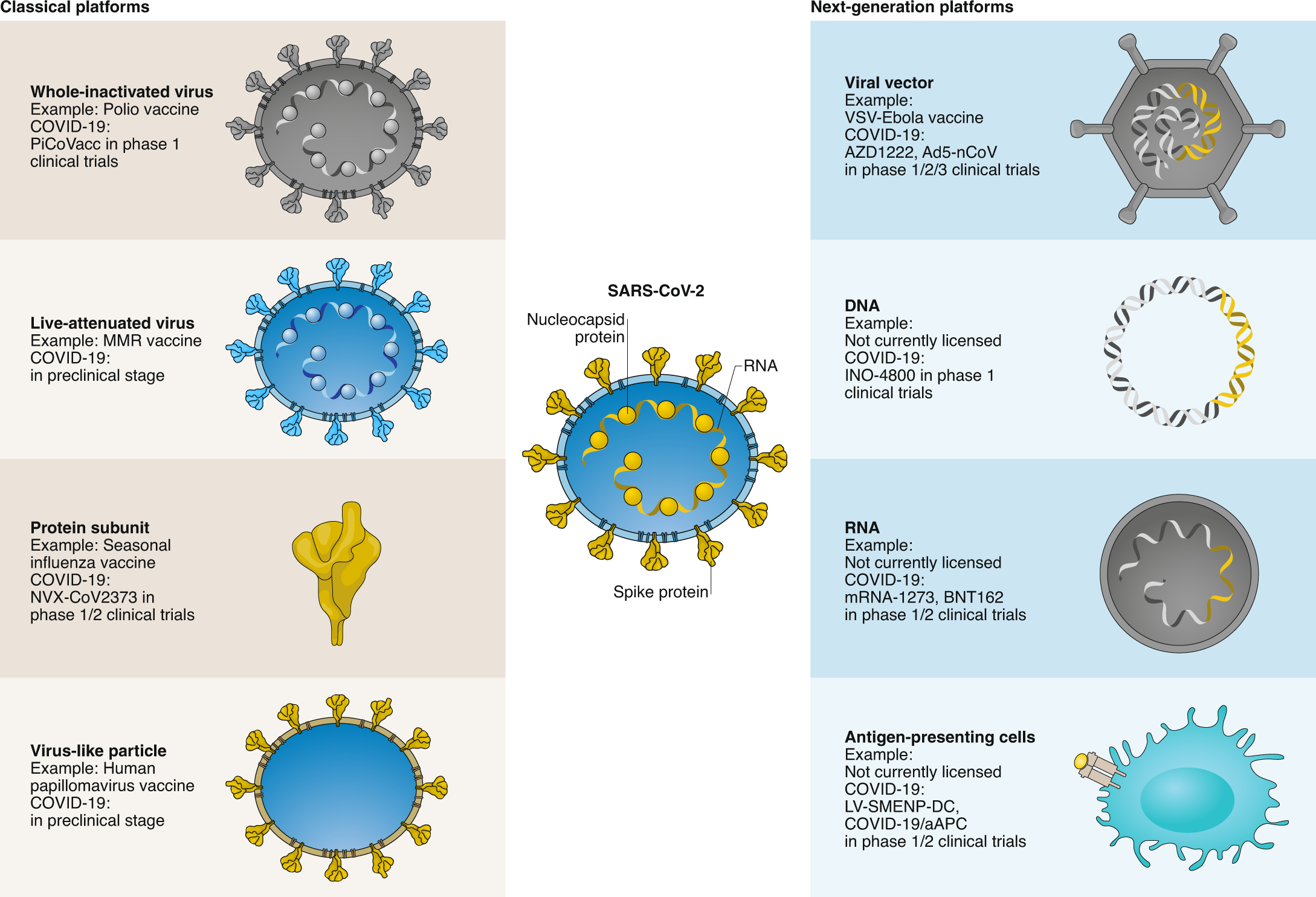
The new nucleic acid-based vaccines, including mRNA, recombinant viral vector, and recombinant DNA plasmid vaccines, are next-generation platform technologies designed to respond rapidly to new pathogens, bioterrorism threats, and other diseases. The main advantage of these platforms is that vaccines can be developed based on genetic sequence information alone.
Think of it as a train platform that is already built, allowing people to board the train and be transported to their destination. In this analogy, the platform and trains are the new vaccine technologies, and the people are nucleic acids that code for specific proteins from disease-causing agents, such as the spike protein from the SARS-CoV-2 virus. The new technologies shave off years from vaccine development because once a genetic sequence for a pathogen is known, a new vaccine can be developed rapidly.
On January 10, 2020, China shared the genetic code for the new-to-humans SARS-CoV-2 virus, allowing scientists to determine the genetic information coding for the 3831 character spike protein. The coronavirus spikes are like keys that fit into a lock on our cells, opening the door and allowing entry where the virus can replicate. If we could train our immune system to neutralize spike proteins, we could prevent or limit infection from taking hold. With the genetic code for the spike protein in hand, the race for vaccines began. According to this coronavirus vaccine tracker, there are at least 64 vaccines in clinical trials at various stages as of writing this piece.
A concerned community
There is a lot of interest in and concern over the new COVID-19 vaccines in the myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) community. Is it safe to get one? Which one is the best for people with ME/CFS? I would not dare to answer these questions here – as they are best answered in consultation with a doctor – but rather provide a general explanation of the basic biology on how the new approaches to COVID-19 vaccines work.
Hopefully, ME/CFS clinicians will weigh in more on this discussion as additional information about responses to the different types of vaccines becomes available, especially for people with ME/CFS. Cort Johnson from Health Rising recently wrote a blog collating initial statements about the new vaccines from several ME/CFS practitioners.
Disclaimer: I have no horse in this race. I am not a vaccine skeptic, but I understand that population vaccine safety data might not apply to our patient group, let alone to us as individuals. I also understand that science does not have all of the answers sometimes (understatement). I personally know several people with ME/CFS who link their disease onset or worsening to vaccines, as well as many who seem to tolerate them.
Part II: First, back to biology basics
Understanding the new nucleic acid vaccines requires a basic grasp of the “central dogma,” a fundamental concept in molecular biology. Taking a little time to understand how the different vaccines work may help when making a personal decision about which one (or none at all) might be best for you.
The central dogma describes the various steps that are involved in protein synthesis. Proteins are made from strings of amino acids, called polypeptides, folded into different shapes. They serve various roles, including enzymes, certain hormones, immune messenger chemicals, and tissue growth, maintenance, and repair. The human immune system evolved elaborate mechanisms to identify and eliminate foreign proteins and peptides, such as the spike protein produced by the SARS-CoV-2 virus. The new vaccines are built on these concepts.
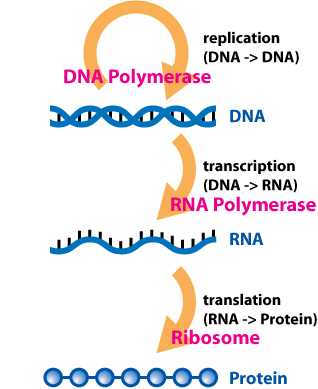
Protein synthesis starts with DNA (deoxyribonucleic acid), which is located in the cell’s nucleus. DNA contains the genetic information that codes for the various traits and functions that make you who you are. Every cell has the same genetic information, but different genes can be turned on or off, allowing cells to differentiate.
Messenger RNA (mRNA) is a type of intermediary messenger that copies instructions from DNA (a process called transcription). The messages are sent out of the nucleus into the cell, telling it which proteins to make.
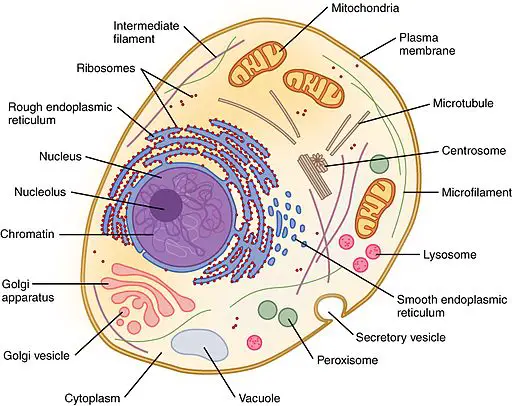

When mRNA leaves the nucleus, it enters the cytosol, the cellular soup holding the various organelles that allow the cell to function, including protein synthesis and assembly. The ribosomes, which are small organelles that stud the endoplasmic reticulum, translate mRNA sequences into polypeptides with the help of transfer RNA (tRNA), creating the building blocks of proteins (a process called translation). Next, the Golgi apparatus processes and packages the proteins for secretion or use by the cell. This is an oversimplification, but it helps to understand the basics of mRNA and recombinant DNA vaccines.
Nucleic acid-based vaccines
Until very recently, vaccines relied on introducing portions of the pathogen to elicit an immune response. In contrast, the new nucleic acid-based vaccines provide a genetic blueprint for our cells to manufacture the spike protein antigen. Regardless of your views on vaccines, the scientific advancements allowing these vaccines are remarkable.
mRNA vaccines: mRNA vaccines have been in development for over a decade but have never been approved for human use until recently with the new Pfizer/BioNTech and Moderna vaccines. First-generation mRNA vaccine approaches were problematic because mRNA proved to be a potent immune activator capable of a severe inflammatory response.
In 2005, the now senior VP at BioNTech, Pfizer’s partner, published a paper showing that modifying mRNA with synthetic nucleosides helps lower immune reactivity and increase protein production (e.g., SARS-CoV-2 spike protein). The new vaccine was designed to reduce (but not eliminate) an exaggerated cytokine response triggered by the activation of toll-like receptors. This is an important point for people with ME/CFS – the mRNA technology was developed to not elicit a harmful innate immune response (but it has in some).

Once injected into the body, the modified, synthetic mRNA is taken up by muscle cells, which use the RNA message to produce spike proteins.
Special immune cells called antigen-presenting cells (APCs) – such as dendritic cells – process the protein as an antigen and present it on its outer surface using the major histocompatibility complex (MHC) system. The APC, with its foreign peptide – in this case, the spike protein – presents the antigen to T- and B-cells to start an antigen-specific adaptive immune response. Eventually, memory B and T cells are made. When challenged with the SARS-CoV-2 virus, our immune system can neutralize the spike proteins, denying the virus entry to our cells or eliminating it if it does.
These vaccines present a few logistical challenges. mRNA is far less stable than DNA and requires packaging in lipid nanoparticles – tiny oily spheres coated in polyethylene glycol (PEG). They have to be stored and transported at ultra-low temperatures.
Recombinant viral vector vaccines. Like the mRNA vaccines, recombinant vector vaccines also offer a “plug and play” approach to vaccine development. This technology uses common double-stranded DNA viruses, such as an adenovirus, to shuttle in genes that code for a specific antigen, such as the spike protein. The only commercial adenovirus vaccine currently available is used to immunize wild animals against rabies. No adenovirus vaccines have been approved for humans until the Oxford-AstraZeneca vaccine, which was recently approved in the United Kingdom and India (but will not be available in the US until spring).
Several of the COVID-19 vaccines use adenoviruses as vectors. Adenoviruses are common viruses that cause mild cold or flu-like illness in immunocompetent people. The Johnson & Johnson, Oxford-AstraZeneca, and CanSino vaccines use genetically-engineered adenoviruses that have been made “replication-defective” by deleting crucial viral genome regions. This prevents the virus from replicating once injected into humans. Each vaccine uses a different adenovirus vector: China’s CanSino uses the Ad5 virus, which has a checkered past in gene therapy and vaccination, Johnson & Johnson uses Ad26, also a human adenovirus, and Oxford-AstraZeneca uses a chimp virus called ChAd. There are many additional types of viral vectors being used to develop the COVID-19 vaccines not covered here.
The reason for using a chimp virus instead of a human virus is that we already have some immunity to human adenoviruses, which might make the vaccines less effective. If there is immunity to the vector being used, booster shots could unleash an immune response against the vaccine itself, which might limit longer-lasting immunity.
This technology was initially designed for gene therapy but failed due to the immense and sometimes fatal immune response caused by the high doses required. As with the mRNA vaccines, scientists learned many lessons on how to fine-tune the immune response. Several vaccine trials are underway for HIV, malaria, Ebola, and even the flu and the therapy has also been used to target certain cancers. It is a more tried and true approach compared to the novel mRNA vaccines. Another advantage is that they do not require ultra-cold storage.
These viruses are capable of infecting a wide variety of cells. Once in the cell, the inserted gene expresses the target antigen (spike protein) under the control of a transcription “promoter” that allows for a robust expression of the genes that code for the spike protein. Adenoviruses remain “epichromosomal,” meaning the viral genome does not integrate with the human host genome and does not inactivate or activate oncogenes.
One attractive feature is that adenoviruses’ inflammatory effects mean developers don’t have to use traditional adjuvants, molecules added to conventional vaccines to direct the immune system’s attention to the viral protein. The adenoviruses themselves drive the inflammation, which is kept under control by giving the vaccines at low doses. However, some of the materials in these vaccines are immunogenic.
All genetic vaccines, including mRNA and adenovirus vectors, mimic a natural viral infection by producing viral proteins inside our cells. Traditional vaccines elicit a B-cell response that neutralizes viruses before they enter our cells. But once a cell has been infected, T-cells are needed to fight infections because they have mechanisms that allow them to sense an infected cell (see Figure 7). The adenovirus vaccines provoke a potent T-cell response.
Recombinant plasmid DNA vaccines. DNA vaccines have been explored for decades. Vaccines currently being trialed or draw on the expertise that came from developing new vaccines for other coronavirus outbreaks, such as MERS. They are cheaper to produce and more stable than the mRNA vaccines.
Instead of using a viral backbone to carry genes into human cells, such as with the adenovirus vaccines, the plasmid DNA vaccines involved genetically-engineered plasmids that contain the genes for the spike glycoprotein. Plasmids are a type of circular DNA found naturally in bacteria, such as E. coli, and yeast. Plasmids used in vaccines also have promoter and enhancer sequences that allow for the expression of the proteins once inside of the cell.
These vaccines have a few advantages to the mRNA vaccines. Because DNA is a stable molecule, unlike mRNA, it does not require the challenging cold chain requirements that the Moderna and Pfizer vaccines do. This could bode well for the rural areas and developed countries lacking adequate cold storage. Inovio, among several companies, is trialing this type of vaccine.
Another newer type of recombinant DNA vaccine involves inserting the spike protein gene into a plasmid. The spike proteins are grown in the lab and used to make the vaccine. This is quite different from the mRNA, adenovector, and DNA plasmid vaccines because rather than providing the genetic blueprint, the actual antigen is injected. This is more similar to the more traditional inactivated vaccines. Since 1986, it has been used in the Hepatitis B and human papillomavirus (HPV) vaccines. These vaccines require adjuvants. Novavax recently announced their recombinant DNA vaccine is entering a phase III trial.
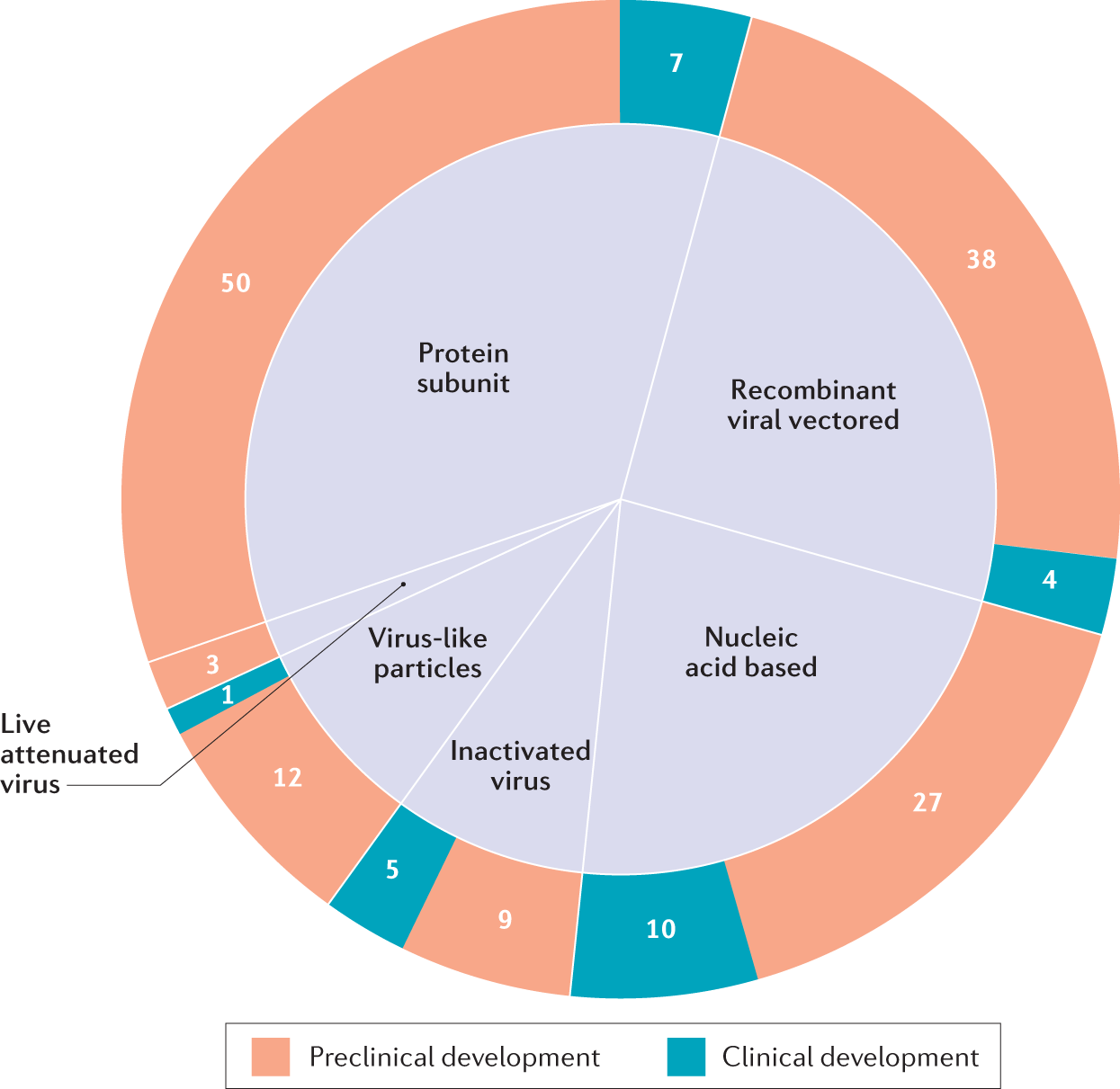
Numerous other companies are using traditional approaches and are at various development stages, testing, and approval. This technical article does a superb job of laying out the different types of vaccines and provides an excellent table that shows the many vaccines under development. The New York Times offers this resource for tracking the status of different vaccines.
Part III: Is it safe?
As people with ME/CFS, we can look at the population-level safety data for reassurance, but ultimately these data won’t tell us how we, as individuals, will respond. There are many immune abnormalities in this illness, but no two patients have the exact same set of impairments. We can look at how others in our community respond to the vaccines, but this has no predictive power for how you might respond.
The safety and efficacy data from phase III trials are available for many vaccines, including those from Pfizer/BioNTech, Moderna, and Oxford/AstraZeneca. Understanding how trial data might apply to an individual is like reading tea leaves. The participants were not stratified for ME/CFS, so we have no data yet on our population’s adverse events. It is possible to look at how people with various autoimmune and neurological disorders responded, but reported events for these groups are small. Immunocompromised people were largely excluded from the trials that have been completed.
It is difficult to assess efficacy data at first glance because each vaccine team used slightly different methods. For example, all UK participants in the Oxford/AstraZeneca trial were required to swab at regular intervals for SARS-CoV-2. In contrast, it is unclear how Pfizer and Moderna referred people for testing, raising concerns about the possible unmasking of participants (these are meant to be blinded studies). The symptoms of COVID-19 and post-vaccine immune activation overlap tremendously – both involve an immune response, making it important to test everyone, regardless of treatment arm.
This opinion article in the British Journal of Medicine provides an overview of some of the gaps in the trial protocols and data and argues that different endpoints are needed, such as the ability to prevent infection. Finally, it is important to remember that the efficacy data were taken over a short period – we don’t know how these vaccines will perform over the longer term.
Despite these issues, the trial data suggest that the vaccines thus far are safe and effective for the endpoints studied, with mostly transient symptoms following vaccination. Symptoms such as malaise, fatigue, muscle pain, and mild fever are signs that the immune system has been activated and the vaccine is working. These side effects, while unpleasant, are to be expected. It is unclear what longer-term effects will be, if any.
As with all vaccines, getting an appropriate immune response is key. Although the new vaccines do not contain any of the traditional adjuvants, there are compounds in the vaccines that might elicit an immune response. In some people, an extreme response leads to anaphylaxis. These are exceedingly rare events; as of 19 December, the United States has seen six anaphylaxis cases among 272,001 Americans who received the Pfizer mRNA vaccine. Anaphylaxis, if it occurs, happens quickly after vaccination and can be treated in a healthcare setting.
The Pfizer and Moderna vaccines wrap the synthetic mRNA in lipid nanoparticles (LNPs) that help carry it to the cell. The LNPs also contain polyethylene glycol (PEG), a compound commonly found in various medications, toothpaste, and shampoos to increase stability and life span. The “PEGylated” LPNs also act as an adjuvant that bolsters the immune response. Although PEG is used broadly in everyday products, it has never been used in vaccines. It turns out that PEG is not biologically inert. One study found that 72% of people have antibodies to PEG. See this article for more information on the immunogenicity of LNPs and PEG. The U.S. National Institute of Allergy and Infectious Diseases (NIAID) and the Food and Drug Administration (FDA) have set up a panel to further explore these concerns.
Several organizations that overlap with ME/CFS have released guidance on the risk and mitigation of allergic reactions to the mRNA vaccines. For up to date information, check the guidance from The American College of Allergy, Asthma, and Immunology (ACAAI) and the Mastocytosis Society. Much of the advice is based on common sense but is helpful. There is no consensus yet from ME practitioners, but some clinicians have begun to make statements.
My personal take
Even though I am mostly at home, I long for the day when I can visit the places I love and be with the people I adore. In the meantime, I will explore the science behind the new vaccines with a mix of wonder and healthy skepticism. I am weighing the vaccine’s potentially negative effects against the uncertainty of how COVID-19 could further undermine my health. It will be many months before I am offered the vaccine, giving me ample time to see what the true adverse event rate looks like once hundreds of millions of people have been vaccinated, including those with various autoimmune conditions and ME/CFS. My hope is that a clear vaccine candidate will emerge.
I am not a vaccine scientist, but I taught much of the basic biology behind vaccines as a tenured professor for over a decade. If I got any of the science wrong, please let me know in the comments.
I have MECFS which was massively exacerbated by the Pfizer vaccine. I got the 2 doses after just coming out of a crash which lasted 6 weeks. I was then labelled as “just anxious and depressed” for one year all while being completely bedridden, with young kids to look after…I am basically useless. I still have abnormal blood tests, extreme fatigue, neuro, cognitive, cardio and respiratory symptoms. I could barely toilet and shower, couldn’t remember names and dates for medical forms. Finally after about 50 Dr visits, tests and hospital trips after collapsing multiple times (extremely low BP) I have found a fantastic specialist who has diagnosed me with post covid vaccination syndrome. Hoping in time, I will “recover” enough to be able to be a mum again. I wish I never got the vaccine. I know they save lives but there has to be a better way 😦
LikeLike
I am so sorry to hear that the vaccines harmed you – I also suspect they caused some of the new health issues I am struggling with. I wish we could have more meaningful conversations in our society about adverse events – not the usual posturing, but a deep scientific discussion about how variation in immune function maps onto vaccine responses. I am so glad to hear you found a doctor who acknowledges your illness and its source – I hope they can offer you some meantinful treatments.
Are you finding any treatments for low blood pressure to be helpful? I am writing a blog on low BP. My cognition has been declining, making writing a bit more of a challenge!
Sending best thoughts xxx
LikeLike
I’m so sorry you have had some issues after the vaccine too. It really is so frustrating when you try to do the responsible and “safe” thing and then suffer immensely. I think in time, more people with side effects will put 2 and 2 together and hopefully the conversation changes. I’m not finding much helps the low BP, I’ve tried various herbal teas, increasing salt, hydrating and those electrolyte drinks (full of sugar as well but they actually make me feel more alive for a few hours!). Sitting upright helps a little but I’m too tired! The specialist has ruled out POTS but has diagnosed “Orthostatic Intolerance ‘.
I’m also seeing a naturopath who is encouraging self care, breathing techniques and focusing on getting vitamins and minerals through nutrition, it has helped a little with my overall wellbeing.
Your article is really informative and well written, I only just found your website yesterday and am looking forward to reading more of your posts 🙂
I hope you have more good days than not x
LikeLike